The FDA has never asserted that the off-label prescribing of ivermectin for COVID-19 was prohibited; the agency has reiterated this point in court and in public filings many times prior to August 2023.
In mid-August 2023, several social media accounts and fringe news outlets began posting the claim that the U.S. Food and Drug Administration (FDA) had recently "admitted" that doctors were now allowed to prescribe ivermectin — the anti-parasitic drug briefly considered to be a possible treatment during the coronavirus pandemic — for COVID-19.
For example, on X, the platform formerly known as Twitter, former Arizona gubernatorial candidate Kari Lake posted that, "The FDA has now admitted that Doctors CAN prescribe Ivermectin to treat COVID." Conservative activist Charlie Kirk posted the same statement, wondering how many lives could have been saved had the admission come sooner.
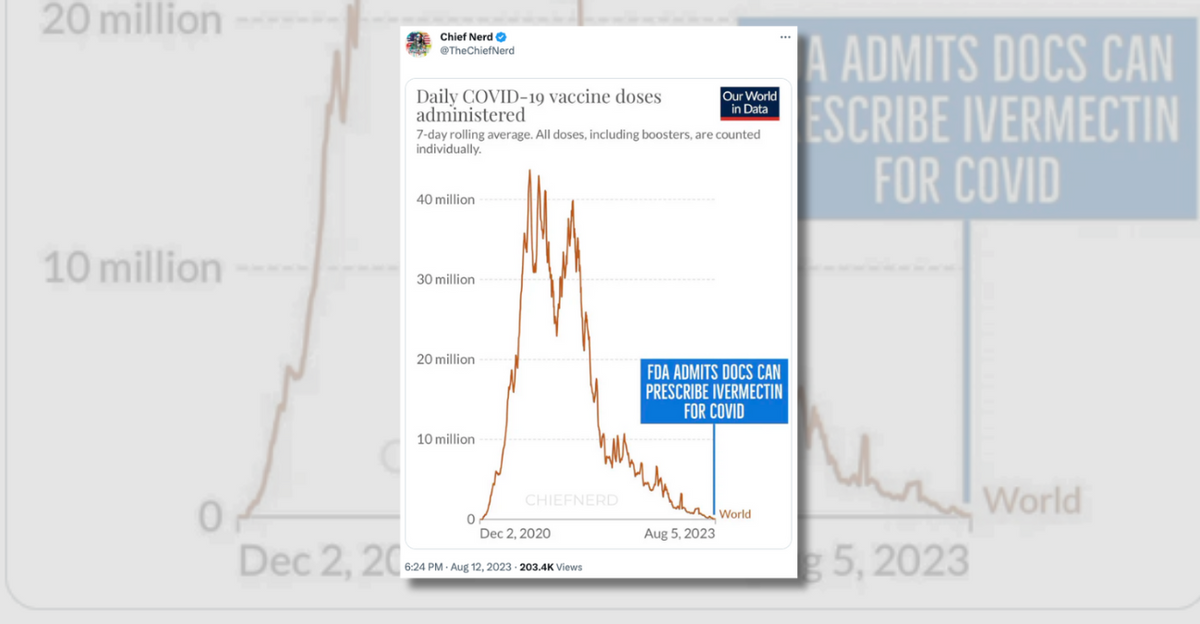
The account "Chief Nerd" posted a chart implying that the purported change in policy coincided with the fact that large-scale efforts to vaccinate people against COVID-19 had passed:
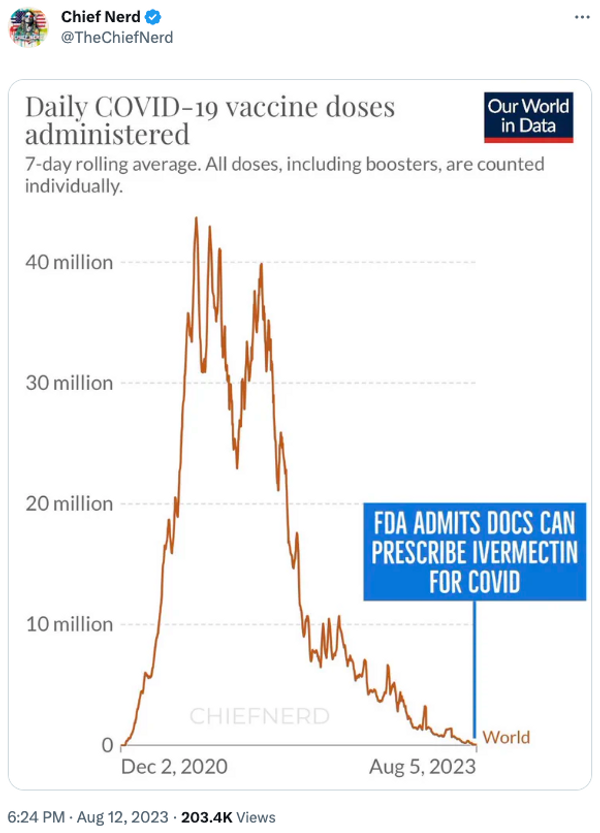
Several flaws existed with these assertions. First, the FDA has never had the authority to prevent doctors from prescribing FDA-approved drugs like ivermectin for off-label uses. The FDA regulates how manufacturers of drugs can advertise and label their drugs to the public and to doctors, but that authority does not extend to regulating how doctors prescribe these medications.
That policy is not new, as exemplified by a 2009 Congressional Research Service report on off-label uses of drugs (emphasis ours):
New drugs may not be introduced or marketed without the approval of the Food and Drug Administration (FDA). When a person submits a drug application to the FDA for approval, the application includes samples of the proposed labeling. The FDA may refuse to approve an application if the drug is not safe or effective for the specific uses that are reflected in its labeling.
An unapproved new use of a drug, also known as an off-label use, is a use not mentioned in the drug's approved labeling. Although a physician may prescribe a drug for off-label uses, a pharmaceutical manufacturer may not market or promote uses of a drug other than those on the label—those uses approved by the FDA in the application.
Claims regarding a purported change in policy, pushed by the outlets Gateway Pundit and Epoch Times, rely on several misconceptions. The first misconception is that the FDA ever prohibited doctors from prescribing the drug for COVID-19 in the first place. Gateway Pundit cited tweets and guidance documents from the FDA as if they were legal directives to make this point.
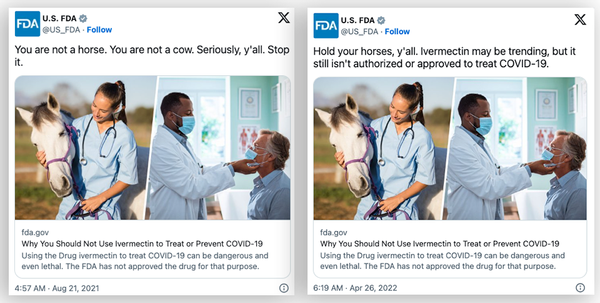
The linked guidance document does not prohibit doctors from prescribing the drug, but it does reiterate that the FDA has not approved the drug for COVID-19 treatment.
The claim of a recent "admission" from the FDA that doctors were "now" allowed to prescribe the drug for COVID-19 stems from a lawsuit filed in federal court against the U.S. Department of Health and Human Services (HHS) by three doctors. They assert their right to practice without federal interference was unconstitutionally limited by the FDA's statements against ivermectin, as described in a legal filing:
Attempts by the FDA to influence or intervene in the doctor-patient relationship constitute interference with the practice of medicine, the regulation of which is—and always has been—reserved to states. In fact, the Federal Food, Drug, and Cosmetic Act ("FDCA") is explicit that it does not in any way authorize the FDA to "limit or interfere" with the practice of medicine, codified at 21 U.S.C. § 396.
The FDA breached this critical boundary between federal and state authority by repeatedly directing the public—including health professionals, professional organizations, and patients—not to use ivermectin for COVID-19, even though the drug remains fully approved for human use.
The doctors who sued initially lost the case in December 2022. They appealed the ruling. Oral arguments for the appellate case were first held on Aug. 8, 2023. A statement made during these oral arguments provided the seed that generated false claims about an FDA policy reversal in August 2023, as asserted by Gateway Pundit:
On Tuesday, a lawyer representing [the] U.S. Food and Drug Administration (FDA) clarified the agency's stance on the use of Ivermectin for treating COVID-19 patients. The lawyer confirmed that doctors have the authority to prescribe the drug [...] for off-label use in treating COVID-19. [...]
During the oral argument, Ashley Cheung Honold, a Department of Justice lawyer representing the FDA, stated that the agency "explicitly recognizes" that doctors do have the authority to administer ivermectin to treat COVID.
"FDA explicitly recognizes that doctors do have the authority to prescribe ivermectin to treat COVID," said Honold.
As this same Gateway Pundit article makes explicit in its own story, this is far from the first time the FDA has "admitted" this fact. In fact, oral arguments made during the initial portion of this same lawsuit provided Gateway Pundit and others with essentially the same claim of the FDA backtracking on ivermectin a year prior, in November 2022:
During a recent hearing, government lawyers argued that the Food and Drug Administration (FDA) was only giving advice and it was not mandatory when it told people to "stop" taking Ivermectin for COVID-19. [...]
"The cited statements were not directives," said Isaac Belfer, one of the lawyers. "They were not mandatory. They were recommendations. They said what parties should do. They said, for example, why you should not take ivermectin to treat COVID-19. They did not say you may not do it, you must not do it. They did not say it's prohibited or it's unlawful. They also did not say that doctors may not prescribe ivermectin."
Statements in this legal proceeding are not the only place in which the FDA has publicly stated that off-label use of ivermectin is allowed. An October 2020 guidance reaffirms this right specifically as it applies to potential COVID-19 therapies:
Once the FDA has approved a drug for a disease or medical condition, health care providers generally may prescribe or administer the drug in clinical practice for an unapproved use not described in the approved labeling (i.e., "off-label") based on their medical judgment, recognizing that the FDA has not assessed the safety or effectiveness of such use.
An April 2023 version of that document reaffirmed this same right, as well.
Because the FDA never had — or even asserted that it had — the authority to block off-label use of FDA-approved drugs, and because the FDA had repeatedly reaffirmed that fact throughout the pandemic, claims that a new policy shift occurred in August 2023 regarding the prescribing of ivermectin for COVID-19 were false.